Which of the Following Atoms Has the Largest Atomic Radius
The third period will have larger atomic radius than the second period since the third period lies below the second period. Li O Cor F.
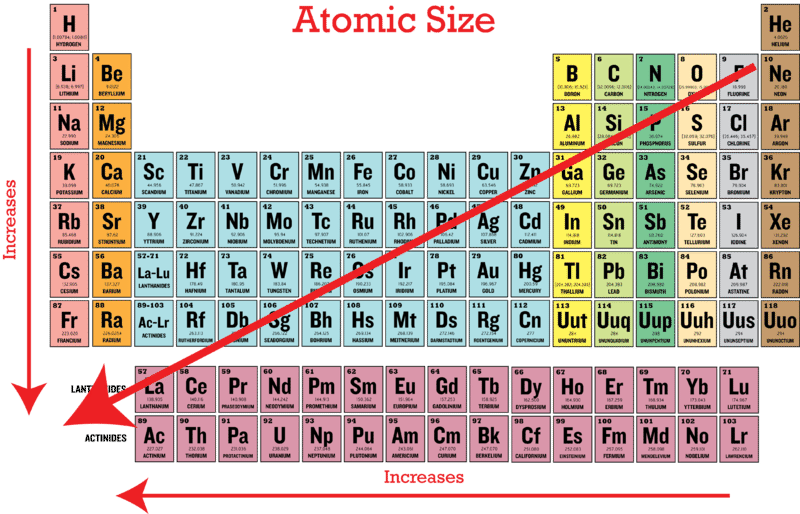
Periodic Trends In Atomic Size Ck 12 Foundation
So an atom of helium is significantly smaller than an atom of hydrogen measuring by the radius of the electron cloud.

. Whereas Na and Cl belong to the third period. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875 Gallium is in group 13 of the periodic table and is similar to the other metals of the group aluminium indium and thallium. Trends in the atomic radius showing that moving down the periodic table size increases whereas moving across the periodic table from left to right size decreases.
As a result orbital electrons become more closer to nucleus. Belongs to the 2nd period. Mg Si and Na which has the smallest atomic radius.
School Tates Creek High School. CL has a larger Atomic Radius. Element Z is larger than Element X.
Which one of the following atoms has the largest radius A Sr B Ca C K D Rb E Na. Atomic radius means the distance between the farthest electron and the center of the nucleus. Based on this you could say.
Question 9 Part A Of the following which atom has the largest atomic radius. Pages 4 This preview shows page 1 -. Atomic radius increases Select and decreases from Select across a row in the periodic table.
C Element Z and X are probably in the same group. A O B F C S D Cl E Ne Answer. 72 48 Which one of the following has the smallest radius.
Atomic radius and ià nico Section 8-3. Br I K Rb. Na KMg or Ca.
Therefore rubidium has the largest atomic radius whereas helium has the smallest. The melting point of R b B r is 6 8 2 o C while that of N a F is 9 8 8 o C. Hence Sulphur has the largest atomic radii.
Atomic radius increases Select V a column. Br I K Rb. Na and Cl.
Which one of the following atoms has the largest. A Element Z is further to the left side of the periodic table. This is mostly because the charge of the helium nucleus is twice as big as that of the hydrogen nucleus.
Therefore according to the trend Cl has a larger radius because it is lower in the table. Atomic radius is half the distance between adjacent atoms of the same element in a molecule. In general the atomic radius decreases as we move from left to right in a period and it increases when we go down a group.
Which of the following atoms has the largest atomic radius. Which of the following elements has the largest atomic radius. Out of the following atom with largest atomic radius.
Chemistry questions and answers. Option c is correct. Question 9 Part A Of the following which atom has the largest atomic radius.
Which among the following element has largest atomic size. Hence K has the largest atomic radii. K will have largest atomic radii.
D A andor B. Moreover Na lies to the left of the periodic table and Cl lies to the right of the periodic table. Incomplete electronic shells shield the nuclear charge VERY INEFFECTIVELY with the result that atomic.
ALONG GROUP-- it increases. A Na B. As the nuclear charge increases the attractive force between nucleus and electrons increases.
The atomic radii increments on going up to down in a group with an increase in a number of shells and decreases along a period as effective nuclear charge increases. The size of an atom is reasonably the radius of its valence electron s. Course Title PHYSICS physics.
In periodic table 3rd period will have larger atomic radius than the second period. E B andor C. AN BO CF DP ES Your answerD Explain your answer.
Therefore Select has the largest atomic radius. TENDONS ON THE TABLE SECTION PERÃDICA 8-1. Answer 1 of 4.
O2z1qpv and 9 more users found this. Atomic size INCREASES down a Group a column of the Periodic Table. Which of the following elements has the largest atomic radius.
Which of the following elements has the smallest atomic radius. Helium has an atomic radius of 31 pm hydrogen has an atomic radius of about 53 pm. In determining the radius of an atom we have to consider the number of protons and the how many shells does this atom have.
Helium has an atomic radius of 31 pm hydrogen has an atomic radius of about 53 pm. Francium has the largest atomic radius since this atom contains the maximum number of shells with the lowest number of protons. Belong to the 3rd period.
An atom or group of atoms that has a positive or negative charge. Which of these atoms has the largest atomic radius ar cl mg na. Chemistry questions and answers.
1 hour agoGallium is a chemical element with the symbol Ga and atomic number 31. Again Cl lies to the right and Na lies to the left of the. Elements Z and X are compared.
Elemental gallium is a soft silvery metal in standard temperature and pressureIn its liquid. Section of group names 8-2. This is mostly because the charge of the helium nucleus is twice as big as that of the hydrogen nucleus.
In is defined as size of on atom or distance from nucleus to outermost shell of an atom. Li 3 21 KL Na- 11 281 K L M. For example- Consider 1st group.
As you go down the table more electron shells are added to make the radius larger but as you go to the right the protons attraction increases so the radius actually shrinks. Na has the largest atomic radius. And ii shielding by other electrons.
Any process that results in the. This radius is i a function of Zthe atomic number. B Element X is closer to the top of the periodic table.
Affinity of ionization and affinity of electrons Chapter 8 Practice and review of quizzes. So an atom of helium is significantly smaller than an atom of hydrogen measuring by the radius of the electron cloud. So atomic radius decreas.
The principal reason that the melting point of N a F is much higher than that of R b B r is that. Hence Na has the largest atomic radius of the above-mentioned elements. Li O Cor F.
Which of the following elements have the largest atomic radius. From left to right in a period atomic radius gradually decreases due to increase in nuclear charge.
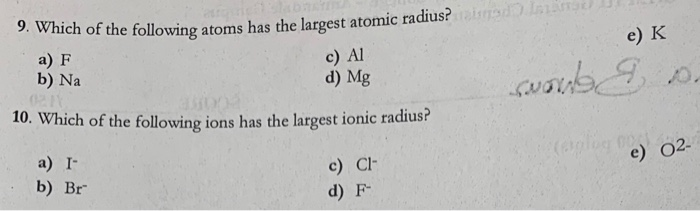
Solved Which Of The Following Atoms Has The Largest Atomic Chegg Com
Atomic Radius Trend Periodic Table Chemtalk

No comments for "Which of the Following Atoms Has the Largest Atomic Radius"
Post a Comment